Thermodynamics » Gases
Gases
A gas is a substance that takes up the shape and volume of the container into which it is placed. It is made up of huge number of molecules which move about freely and at different speeds in straight lines. These molecules are at constant motion and this knowledge of gas behaviour is important in designing internal combustion engines.
Laws of Gases
The gas laws were developed to understand that the interactions between pressure, volume and temperature of a gas can be obtained to hold to a good approximation for all gases.
Boyle’s law: Robert Boyle stated that the pressure exerted by a gas held at a constant temperature varies inversely with the volume of the gas. That means, for a fixed mass of gas at constant temperature, the volume is inversely proportional to the pressure, which is stated as a formula below.
PV = constant
P = pressure
V = volume
Thus the pressure is increased with the decrease in volume and vice-versa. This relationship paves way to calculate the new conditions of pressure and volume, as shown below.
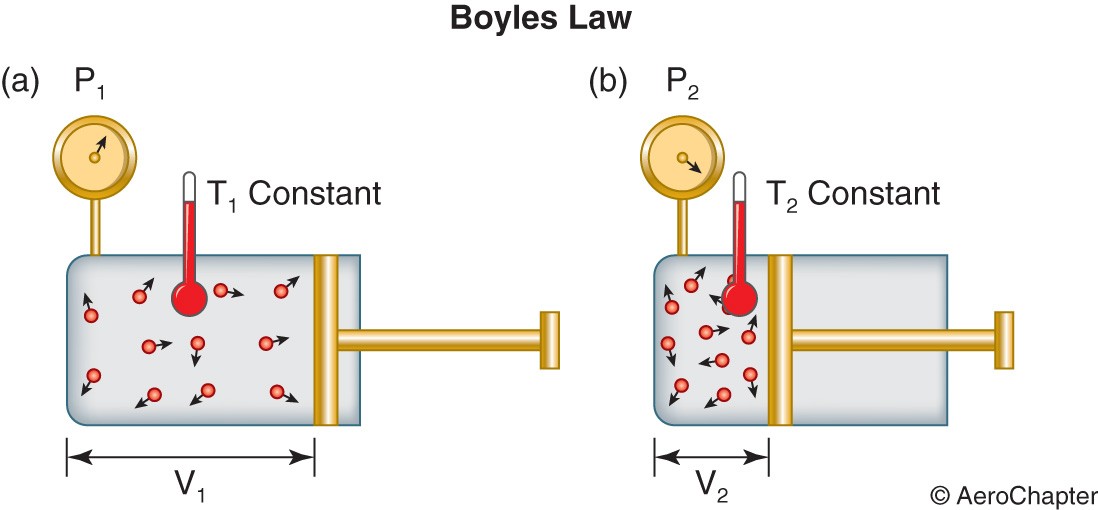
P1V1 = P2V2
P1 = pressure at piston position 1
P2 = pressure at piston position 2
V1 = volume at piston position 1
V2 = volume at piston position 2
Charles’s law: Jacques Charles studied the relationship between the volume and temperature of a gas at constant pressure. He noted that for ideal gas at constant pressure, the volume is directly proportional to its temperature.
T = temperature
As shown in the following figure, if heat is applied to the cylinder, the piston must be moved out in order to maintain constant pressure. Similarly if the temperature is lowered, the piston must be moved in to maintain its original pressure.
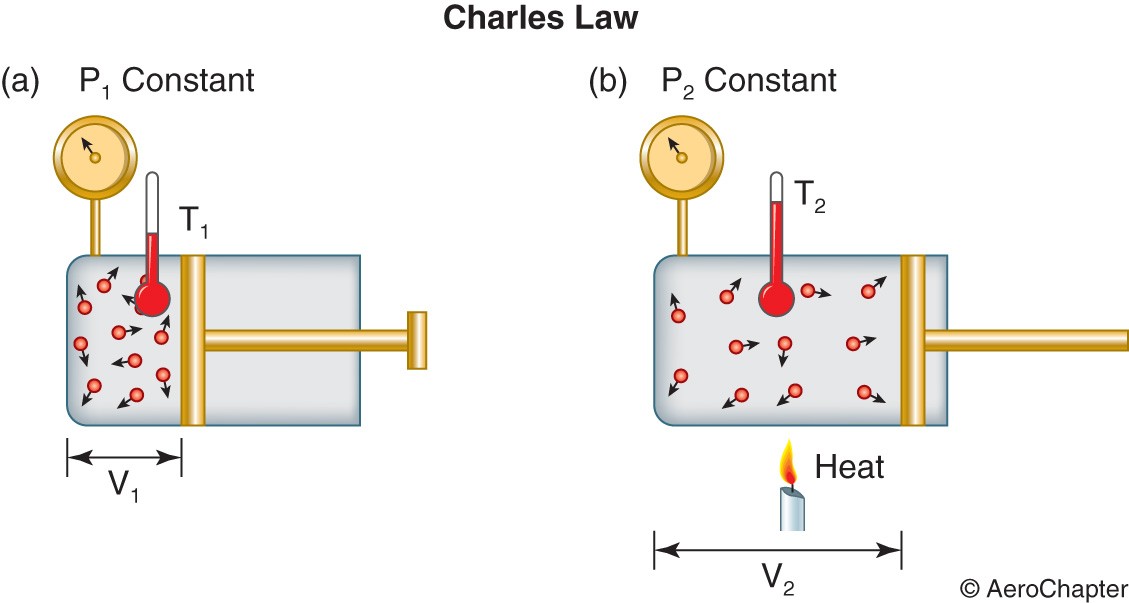
T1 = temperature at piston position 1
T2 = temperature at piston position 2
Boyle’s law and Charles’s law together is called as the combined gas laws. For this, a fixed mass of gas must be allowed to expand at constant temperature and constant pressure according to Boyle’s law and Charles’s law, respectively. This is the ideal gas rule or the characteristic gas equation, which is represented as shown below.
PV = mRT
m = mass of the gas (kg)
R = gas constant
Expansion of Gases
A gas requires more heat to produce 1 degree of temperature rise if the volume is kept constant. The reason is that the energy output of the expanding gas must be balanced by an additional energy input in the form of heat. The amount of expansion differs with specific heat values, only under the two following conditions.
- The gas is kept at a constant volume without allowing to expand.
- The gas is kept at a constant pressure whilst the gas is allowed to expand.
The specific heats are specific heat at constant volume (cυ) and specific heat at constant pressure (cp).
