Thermodynamics » Engine cycles
Engine Cycles
A fluid experiences thermodynamic cycle, when it undergoes a series of processes and returns to its original state. For example, an internal combustion engine operates on a cycle by compressing a gas in a cylinder and creates high pressure by igniting it. Then it allows it to expand against a piston driving it forward.
The air when compressed quickly to rise the temperature is known as adiabatic compression. And the gases when compressed slowly to maintain constant temperature is known as isothermal compression.
The expansion of a gas without any exchange of heat energy is known as adiabatic expansion. And the expansion of gas under constant temperature is known as isothermal expansion.
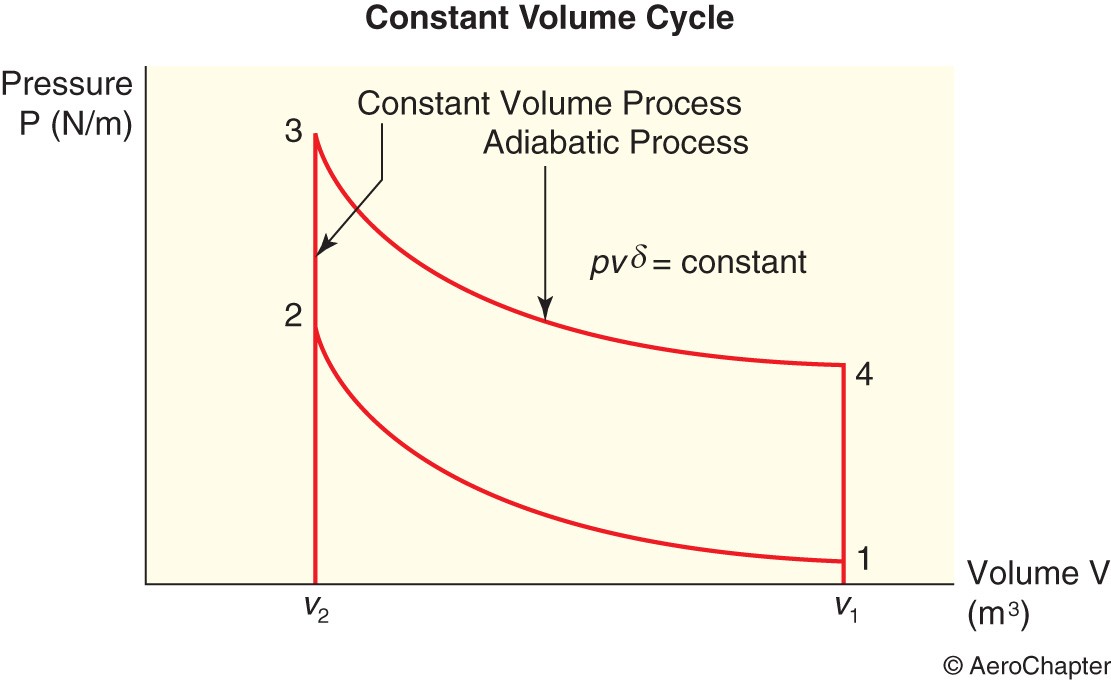
The Constant Volume Cycle
In petrol and gas engines, sudden rise in temperature occurs as the gas is ignited with a little or no movement of the piston at the top centre. And sudden fall in pressure and heat loss occurs with a little or no movement of the piston at the bottom centre. As the exchange of heat takes place whilst the gas volume remains unchanged in both cases, this cycle is known as the constant volume cycle or the Otto cycle.
The Constant Pressure Cycle
This cycle is also known as the Brayton cycle. In gas turbine engines, the heat supply and heat rejection processes occur at constant pressure. The work input to the compressor occurs from the turbine in between 1 and 2. The heat is supplied from the heater between 2 and 3. The work output from the turbine occurs in the form of thrust in between 3 and 4.
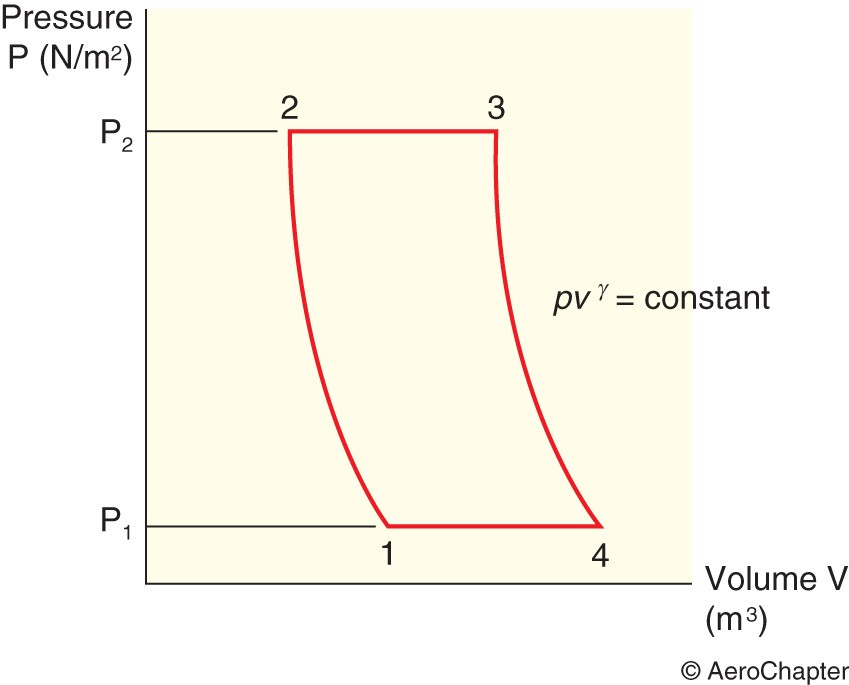
For both the constant volume and the constant pressure cycles, air (ideal gas) is the working substance for an ideal cycle and γ is constant and equal to 1.4.
The Air Standard Cycle
This is a true thermodynamic cycle, in which the working fluid is considered as a fixed air mass that undergoes a full cycle. Heat is added at constant volume in order to produce the rise in pressure at combustion stage. On the other end, heat is extracted from the air, enabling it to return to its original condition before the cycle is repeated.
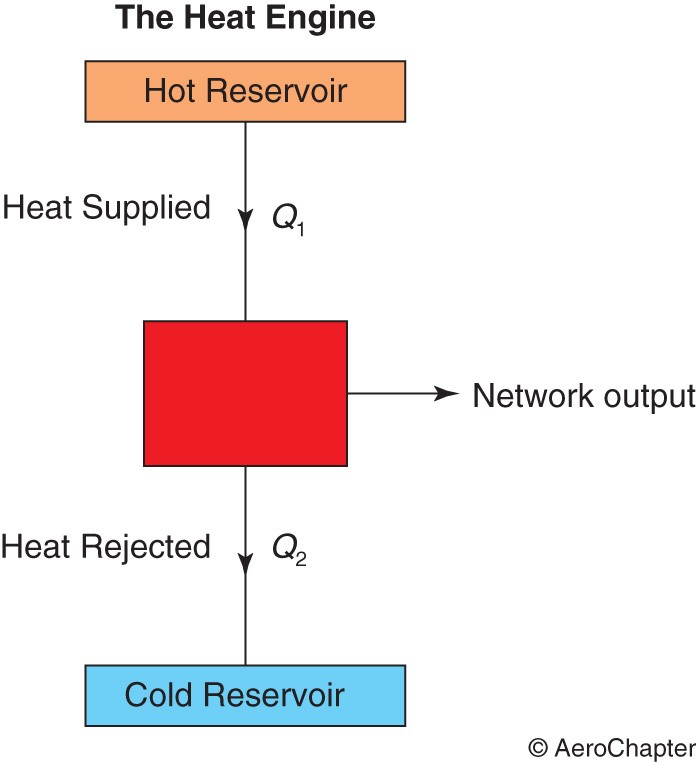
The Heat Engine
It is a system which operates on a complete cycle and develops a network output from a supply of heat, as shown in the diagram below.
Based on the first law of thermodynamics, the following equation is derived.
Q1 – Q2 = W
(Q1 – Q2) = net heat supplied
W = net work done
Based on the second law of thermodynamics, the following equation is derived.
Q1 > W
The thermal efficiency (η) must always be less than 1, according to the formula derived below.
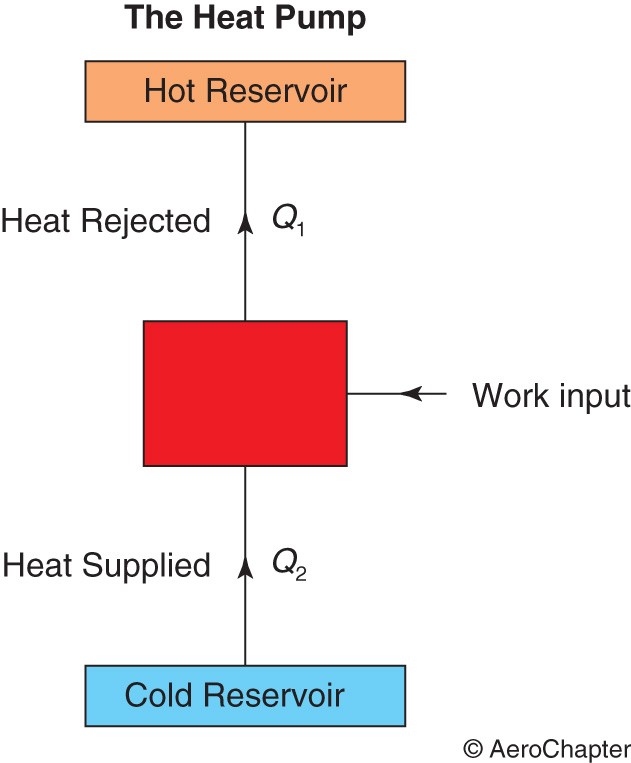
The Heat Pump
This system operates on a cycle that works in the reverse direction to that of the heat engine, as shown in the figure below.
Based on the first law of thermodynamics, the following equation is derived.
Q1 = Q2 + W
Based on the second law of thermodynamics, the following equation is derived.
W > 0
Heat pumps are used in the heating of buildings with the evaporator placed in the air outside the building and the condenser being placed inside the building. The heat pump transfers heat from the medium outside to inside the building with the condenser. Some electronic equipment on military aircrafts have to be heated at altitude in order to maintain a good working temperature. These use a heat pump system which automatically cools or heats depending on the temperature of the electronics bay.
