Atmosphere » Temperature
1.5 Temperature
Temperature is the measure of average kinetic energy of the molecules of a substance.
Units
The most commonly used temperature scales are Celsius or Centigrade, Fahrenheit and Kelvin. The first two scales are based on the melting point of ice, being 0°C and 32°F respectively, and the boiling point of water, being 100°C or 212°F. Being a form of energy, heat is related to the random movement of molecules in a substance. If heat is reduced, the molecules become less active. The minimum temperature to which a substance can be reduced is approximately -273 °C, and this is known as Absolute zero, or 0 K. Correspondingly, the melting point of ice is equivalent to 273 K and the boiling point of water to 373 K.
To convert from one temperature scale to another, the following formulae may be used:
F = y + 32
C =5/9 (F-32)
K = C + 273
summary
- Freezing point of water is: 0 0C or 32 0F or 273k
- Boiling point of water is: 100 0C or 212 0F or 373k
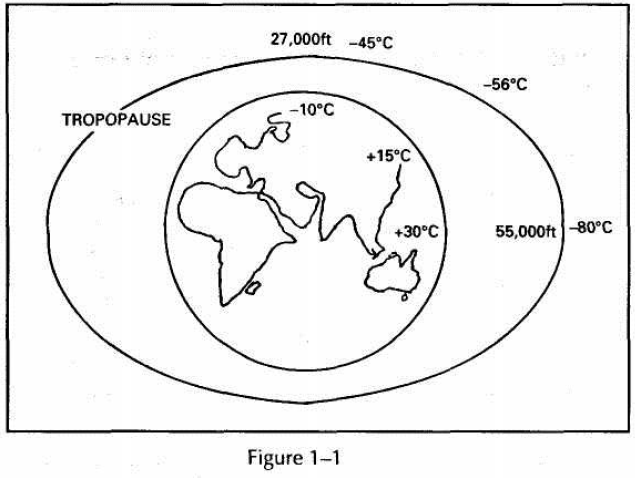
Temperature Variation in the Troposphere
At ground level, in general, the temperature increases with decrease of Altitude.
With increasing altitude, the conductive and convective effects from the earth are reduced so that the temperature will usually decrease with height up to the tropopause. See Fig. 1-1
Typical values of temperature found at the tropopause are:
Altitude Temperature
Equator -80°C
45°N/S -56°C
Poles -45°C
There is, therefore, a reversal of temperatures with Altitude in comparison to those found at ground level. This is partly because the tropopause is higher at the equator and the temperature decrease is effective over a greater height.
Lapse Rates
The rate of temperature decrease with increase of height is referred to as lapse rate.
A representative value of 2°C/1000 ft is a typical value for the troposphere, and this figure is used as the reference for the Jet Standard Atmosphere (JSA).The International Standard Atmosphere (ISA) uses the comparable value of 1.98°C/1000ft. For meteorological purposes, differentiation between dry (that is, not saturated) and saturated adiabatic lapse rates is made, and the values of 3°C/1000 ft and 1.5°C/1000 ft respectively are used. The difference of lapse rate for saturated air is caused by the release of latent heat during condensation, thus reducing the temperature change.
Temperature and Aircraft Performance
At a given pressure, an increase of temperature results in a reduction of density. Firstly, considering airframe performance, a reduction of density (p) reduces lift (L). This may be counteracted by increasing the true airspeed (v) to achieve the required amount of lift (L):
L = CL ½ pV2S
where: CL = coefficient of lift Thus: CL Vi pV2S
and S = surface area
The dynamic pressure is gained at the expense of an increased take-off run, cruising TAS or landing run according to the stage of flight. On the credit side, drag (D) reduces with increase of temperature:
D = CD ½ pV2S
A piston engine's performance is related to the temperature of the air being drawn into the cylinder head. The higher the temperature, the lower the density and weight of fuel/air mixture that can be burnt in the combustion chamber. The power output of the engine therefore falls with increase of temperature.
For a propulsion system, piston or jet,
Thrust = Mass of air x Acceleration to which air is subjected
Thus an increase in temperature will reduce the mass flow and, therefore the thrust.
