Atmosphere » Gas laws
1.9 The Gas Laws
Whilst air is not an ideal gas, it does conform, within close limits, to the results of Boyle's and Charles' laws.
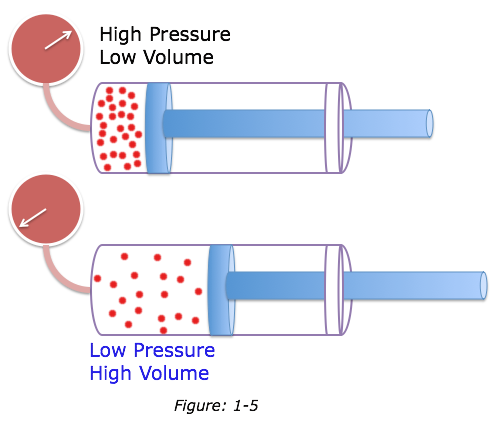
Boyle's Law
The volume (V) of a given mass of gas at constant temperature is inversely proportional to pressure (P):
V = 1/P or PV = constant
This can be expressed in the form:
P1 V1 = P2V2
Charles' Law
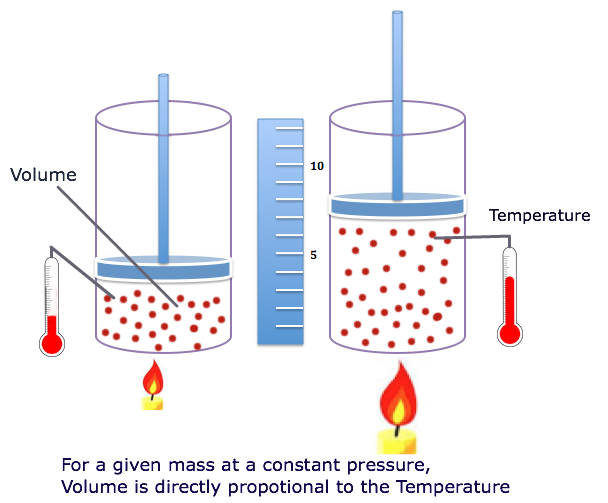
Charles' Law states that at a constant pressure the volume of an ideal gas is directly proportional to its absolute temperature.
The volume of a given mass of gas at constant pressure, increases by 1/273 of its volume at 0°C for every 1°C rise in temperature:
V α T
or
V/T = k (Constant)
Charles' law also be expressed as:
V1/T1 = V2/T2
Combined Boyle's and Charles' Law Equation
The results of both laws may be combined in one equation, expressing the behaviour of a gas under varying conditions of pressure, volume and temperature:
P1V1/T1 = P2V2/T2
Where
P1 = Initial Pressure
V1 = initial Volume
T1 = Initial absolute temperature
P2 = Final pressure
V2 = Final volume
Note: Temperatures are absolute temperatures measured only in Kelvin.
